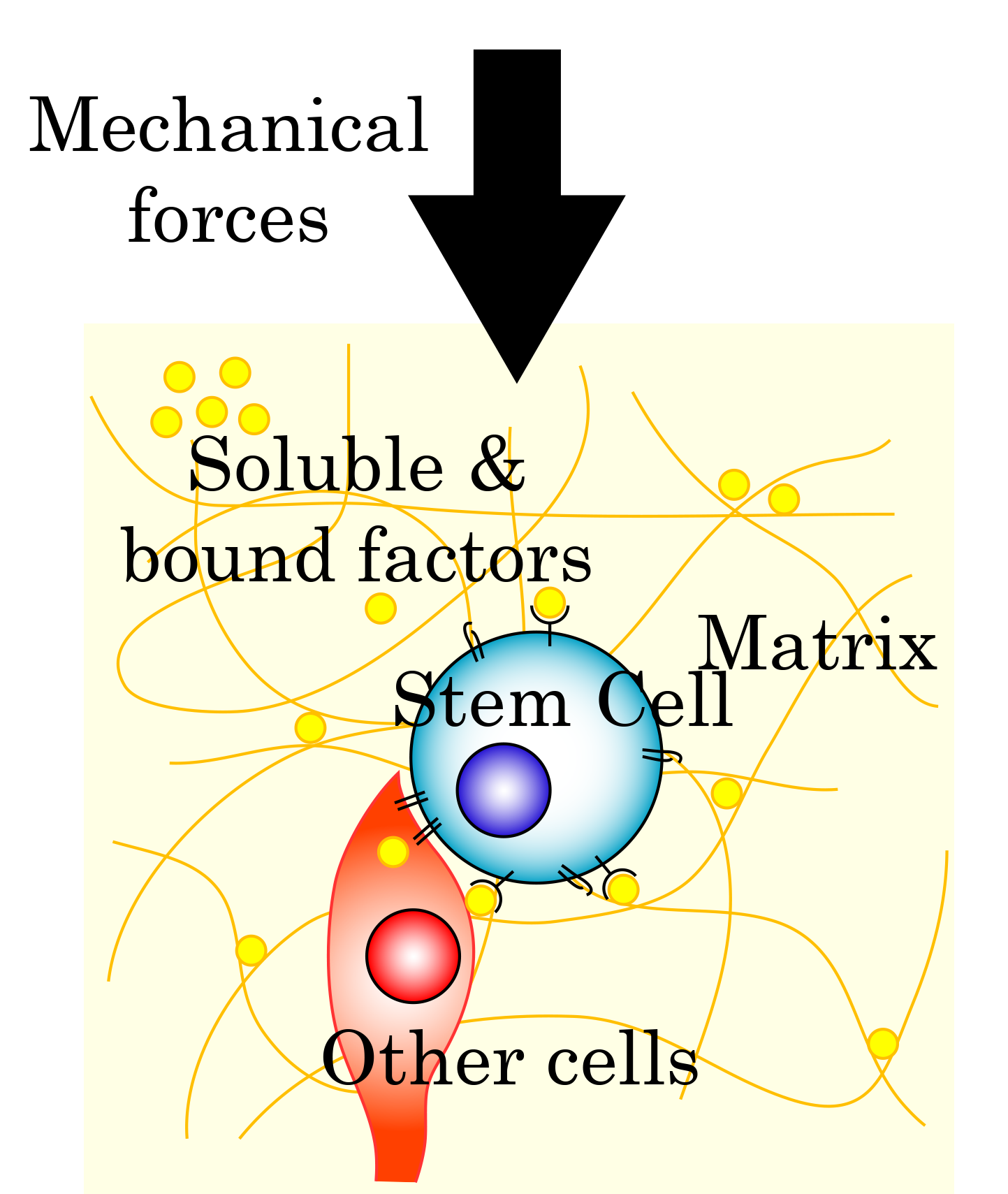
Engineering the Stem Cell Niche
We are interested in the microenvironmental cues that modulate stem cell function and incorporating them into engineered systems. We are specifically studying how these cues can affect adult stem cell proliferation, adhesion, migration and differentiation. We are currently studying how biomaterial-based scaffolds can be used for regenerative medicine with a focus on the nervous system and wound healing. A main specialty of our group is covalent immobilization of molecules to guide cell function. This includes growth factors (to control differentiation), guidance molecules (to steer axons, see below) and fluorocarbons (to modify local oxygen levels).
Protein Engineering
Recombinant modification of proteins with functional groups enables conjugation with numerous small molecules, other proteins or polymers. Further, this approach allows for the formulation of new regenerative strategies for exposing both cells and tissues to proteins, modifying protein mechanisms and presenting these proteins in a highly specific manner.
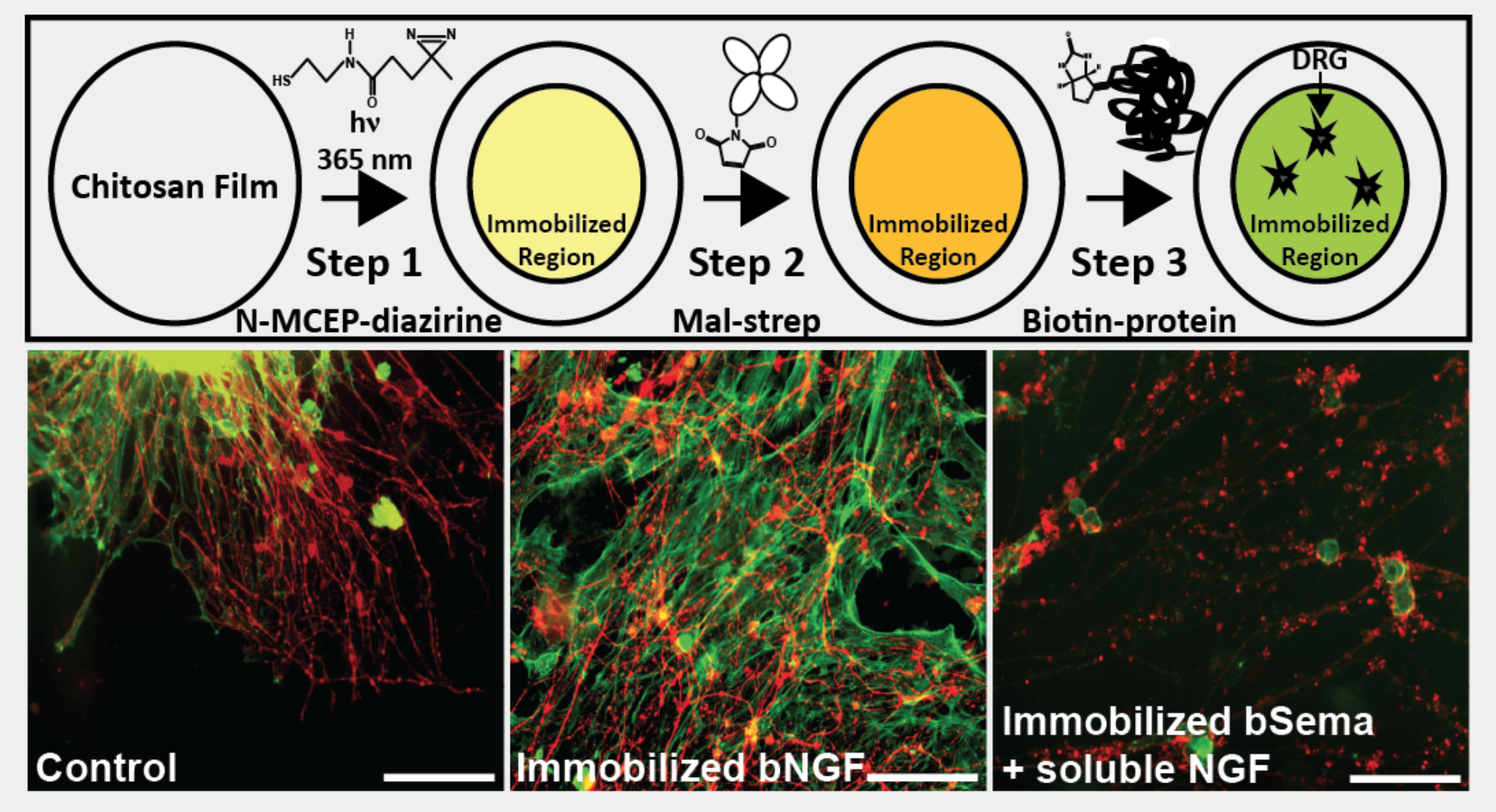
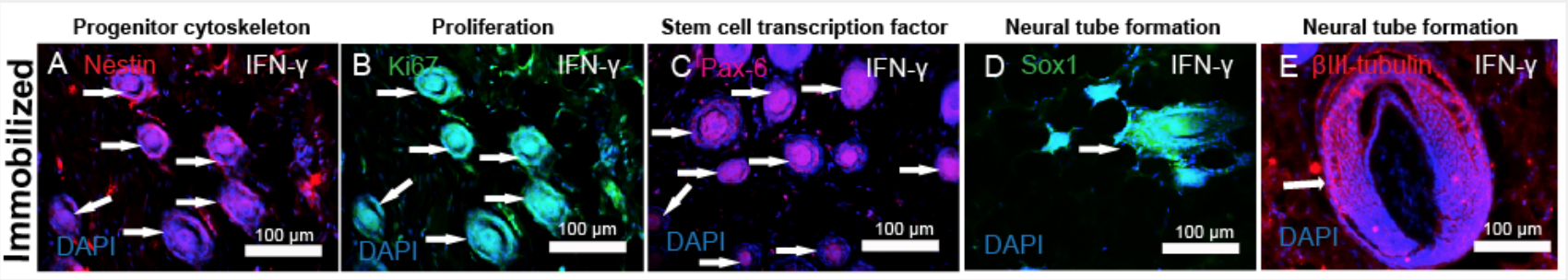
CNS Injury, Repair and Regeneration
Decades of research have yet to offer effective clinical strategies for central nervous system (CNS) repair and regeneration. Previous work has revealed that delivery of a single drug, vector, cell type, etc. does little to mitigate the progression of secondary injury even in animal models. Thus, multi-faceted approaches are needed to address the complexities of CNS injury and, more specifically, spinal cord injury (SCI). Protein sequestration by the ECM is a well conserved mechanism, which can significantly enhance growth factor signaling, allow storage of factors to recruit cells or to facilitate rapid biomolecular responses to events such as tissue scale trauma. We are working on commandeering these endogenous mechanisms, and covalently sequester growth factors to unique biomimetic biomaterial scaffolds we have developed. These approaches will enable localized and enhanced protein signaling to create and sustain regenerative environments in the injured spinal cord when combined with either delivery of exogenous stem cells or soluble release of homing factors to recruit endogenous stem cell populations Formulating strategies to not only recruit but then also direct differentiation and patterning of these cells would be transformative and extend well beyond treating the CNS. Thus, the complexity of SCI demands that new multidimensional strategies be formulated to achieve successful repair and regeneration interventions.
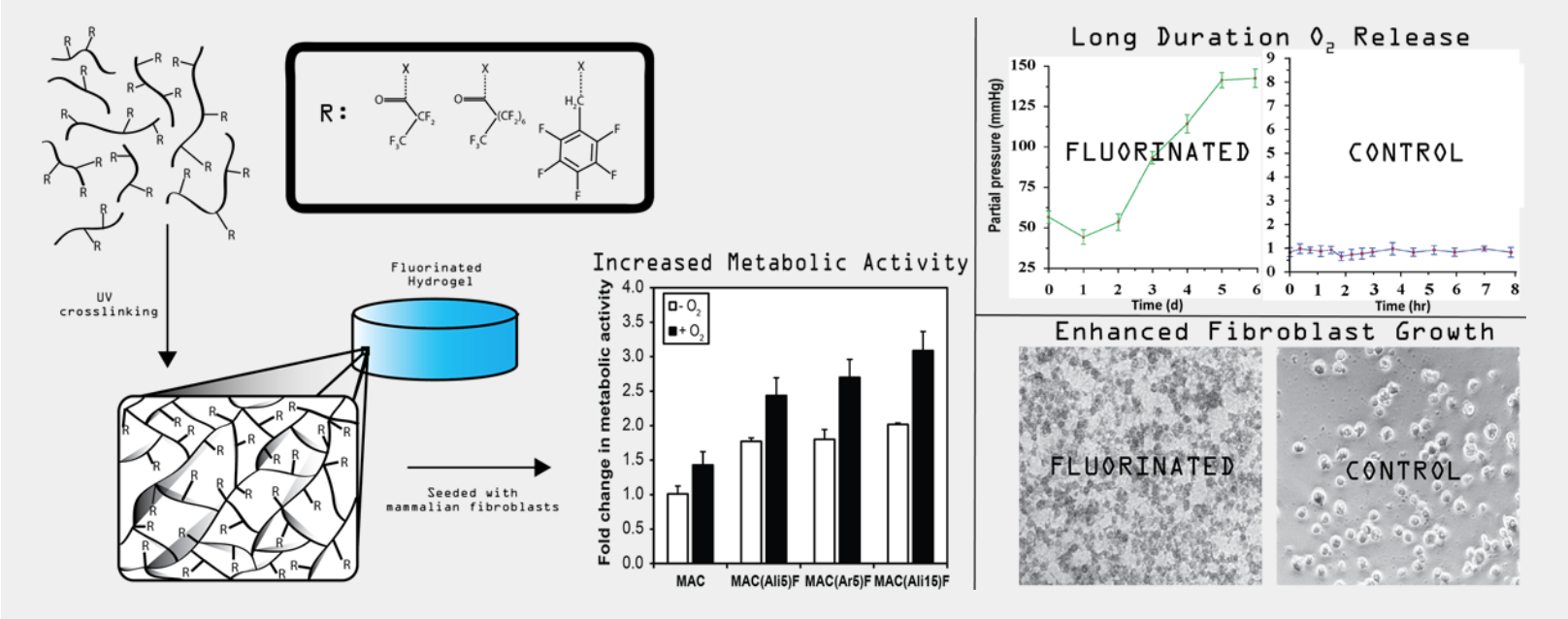
Wound Healing
Oxygen plays a significant role in wound healing, and evidence shows that elevated tissue oxygenation levels are an important factor in the dermal healing response. Specifically, non-healing diabetic chronic wounds (i.e., lasting more than 6 weeks) often show devastatingly low oxygen concentrations, as low as 5mmHg oxygen partial pressure (PO2). In the USA, there are more than 6 million diagnosed chronic wounds with 3-4 million new diabetic ulcers occurring every year, and these numbers are increasing as our population ages. Up to 25% of all diabetics will develop a foot ulcer, and a fifth of these cases will result in a chronic non-healing wound that requires amputation. Oxygen treatment has been demonstrated to promote chronic wound healing by enhancing metabolism, extracellular matrix synthesis, and neo-vascularization, all while limiting antimicrobial activity.
We invented a biocompatible photocrosslinkable chitosan hydrogel covalently modified with perfluorocarbons (PFCs) and can formulate dressings easily from it. PFCs can uptake significant amounts of oxygen and release it to areas of low oxygen tension; however, up until now, PFCs had to be formed into colloidal suspensions to be utilized in medical applications. Our sheet hydrogel dressing covalently incorporates PFCs for sustained oxygenation for up to five days at significant oxygen partial pressures (PO2). Thus, this approach marries the benefits of oxygen treatment with hydrogel dressings (increased angiogenesis, enhanced autolytic debridement, increased re-epithelialization) by providing an oxygen-rich and moist healing environment that is anti-microbial and anti-bacterial.